The field of organ transplantation has taken a significant leap forward with the approval of the first clinical trials for pig-to-human kidney transplants by the US Food and Drug Administration (FDA). This breakthrough marks a crucial step towards addressing the severe shortage of donor organs, potentially offering hope to thousands awaiting transplants. The trials will involve genetically modified pigs designed to reduce rejection risks and ensure safety for human recipients.
Background on Xenotransplantation
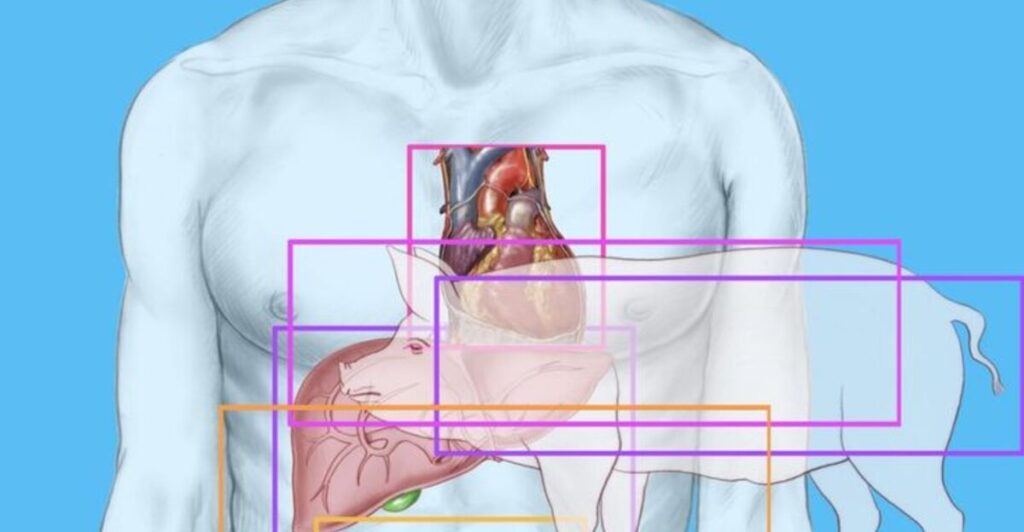
Xenotransplantation is an experimental process involving transplanting organs across species. In this context, it involves transplanting genetically modified pig kidneys into humans. While there have been previous instances of xenotransplants under compassionate use programs, these new trials are formalized and standardized, aiming to provide critical safety and efficacy data.
FDA Approval

The FDA’s approval is a significant milestone in medical research. Two biotechnology companies, United Therapeutics Corporation and eGenesis, have been cleared to conduct these clinical trials using gene-edited pig kidneys for human transplants. This development could revolutionize organ transplantation by providing an alternative source of organs.
Trial Objectives
The primary objective of these trials is to assess whether pig kidneys can be safely transplanted into living individuals with chronic kidney disease whose organs no longer function independently. The focus will be on evaluating the safety and efficacy of such procedures.
Participants in Trials
United Therapeutics plans to enroll six patients initially who have been on dialysis for at least six months without other serious health issues. These participants will be closely monitored for adverse events and long-term outcomes. eGenesis will start with three patients who are unlikely to receive a human transplant within five years.
Monitoring Process

Patients in both trials will undergo rigorous monitoring post-transplantation. They will be observed for 24 weeks initially and must agree to lifelong follow-ups. This monitoring includes tracking health outcomes and potential pathogens that could transfer from pigs to humans.
Genetic Modifications in Pigs

Both companies use genetically modified pigs but differ in their approaches. United Therapeutics employs pigs with 10 genetic edits aimed at reducing organ rejection risks. In contrast, eGenesis uses pigs with 69 edits primarily focused on deactivating viruses integrated into the pig genome.
Challenges Ahead
Despite advancements, several challenges remain. One major concern is managing antibodies produced against pig antigens to prevent organ rejection. Additionally, there are concerns about potential viral infections or other pathogens that could spread from pigs during transplantation.
Previous Compassionate Use Transplants
About half a dozen people worldwide have received xenotransplanted organs from genome-edited pigs under compassionate grounds due to their critical condition or lack of alternatives. However, most did not survive beyond a few months post-transplant largely because they were too ill before surgery began.
Future Implications
If successful, these trials could pave the way for larger studies involving more complex organs like hearts—a more challenging area due to its complexity compared with kidneys.
Ethical Considerations

Ethical considerations play a crucial role in xenotransplantation research. Researchers must address concerns about animal welfare while ensuring that any benefits outweigh the risks associated with cross-species transplantation.
Global Impact Potential
Luhan Yang hopes this FDA approval encourages regulatory agencies worldwide—especially those in Europe and China—to consider similar studies. Such global coordination would accelerate progress toward making xenotransplanted organs available as viable options internationally.
Will Pigs Solve the Organ Crisis?

While it remains uncertain if xenotransplantation can fully solve the global organ shortage crisis immediately after initial success stories emerge from ongoing clinical trial phases conducted by various biotech firms like United Therapeutics Corporation & eGenesis Inc., they undoubtedly represent groundbreaking steps toward addressing one part—kidney shortages—of this multifaceted problem facing healthcare systems globally today.
Discover more of our trending stories and follow us to keep them appearing in your feed
Scientists Are Bringing Back The Wooly Mammoth
First Ferret Babies Born from a Clone—What This Means for the Future
California Is Breaking Apart: A Fault Line Is Forming Faster Than Anyone Predicted
The War on Cows Is Over—And Green Extremists Have Lost
References:
Reference 1
Reference 2
Reference 3
This article first appeared here
Stay connected with us for more stories like this! Follow us to get the latest updates or hit the Follow button at the top of this article, and let us know what you think by leaving your feedback below. We’d love to hear from you!







