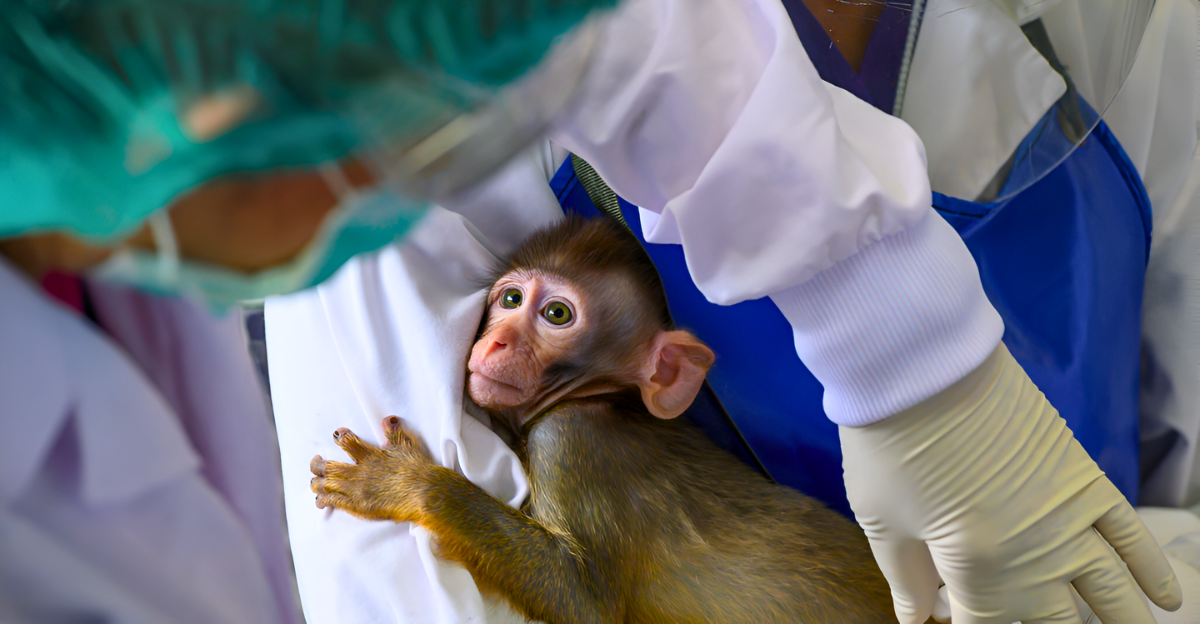
Animal research labs in the U.S. face increasing congressional demands for transparency and reform. Public and legislative pressure is mounting to overhaul animal testing practices in biomedical research, spotlighting ethical concerns and accountability. Despite decades of reliance on animal models, there is growing consensus that full disclosure of animal use data is essential to ensure humane treatment and scientific integrity.
The Federal Animal Research Accountability Act of 2025 exemplifies this shift, aiming to mandate precise reporting of animal use in NIH-funded labs. This reckoning reflects a broader societal push to align research methods with modern ethical standards and technological advances, promising a future where animal welfare and scientific progress coexist more transparently and responsibly.
Legislative Push for Transparency

The Federal Animal Research Accountability Act of 2025, introduced in Congress, seeks to amend the Public Health Service Act to improve the accuracy and transparency of animal use data collected by NIH-funded research entities. This legislation requires detailed annual reporting of animal numbers, species, and pain levels, making the data publicly accessible online.
Bipartisan support underscores the urgency to bring government-funded animal experiments out of the shadows, matching EU, UK, and Canada transparency standards. The Act also includes compliance audits and penalties for non-compliance, aiming to hold research institutions accountable and foster trust among taxpayers and animal welfare advocates.
FDA’s Shift Away from Animal Testing

In April 2025, the FDA announced plans to phase out animal testing requirements for monoclonal antibodies and other drugs, marking a pivotal regulatory shift. This move embraces New Approach Methodologies (NAMs), such as AI-driven computational models, organoid toxicity testing, and in vitro assays, which offer faster, more predictive, and humane alternatives.
The FDA’s strategy is expected to accelerate drug development timelines, reduce costs, and enhance safety assessments while significantly decreasing animal use. This regulatory evolution reflects scientific advancements and ethical imperatives, signaling a new era in drug approval processes.
Congressional Legislation Complementing FDA’s Efforts

Complementing the FDA’s initiatives, the FDA Modernization Act 3.0 has been introduced to enforce the agency’s commitment to reducing animal testing. This bipartisan legislation, supported by key lawmakers, mandates the adoption of validated non-animal testing methods and aligns regulatory frameworks with cutting-edge science.
By legislating these reforms, Congress aims to institutionalize humane alternatives, ensuring that regulatory policies keep pace with technological innovation. This synergy between legislative action and regulatory reform is crucial for accelerating the transition from animal models in drug development.
Animal Welfare Act Enforcement Challenges

Despite legal protections, the Animal Welfare Act enforcement remains problematic, especially in research and breeding facilities. The case of Stonehenge Kennel illustrates persistent violations despite repeated inspections and suspensions, highlighting systemic enforcement weaknesses.
These ongoing issues raise concerns about the adequacy of oversight and the welfare of animals in labs. Such failures underscore the need for stronger compliance mechanisms and transparent reporting to safeguard animal welfare effectively within federally funded research environments.
Ethical and Scientific Critiques of Animal Testing

Animal testing faces mounting ethical and scientific criticism. Physiological differences between animals and humans often lead to unreliable safety data, causing false positives or negatives in drug trials. Advocacy groups emphasize the moral costs of pain and suffering inflicted on lab animals, questioning the justification for continued use.
This growing critique fuels the push for more humane, scientifically advanced alternatives that promise better predictive accuracy and ethical standards, reflecting a paradigm shift in biomedical research ethics.
Technology and Drug Development

Emerging technologies like organ-on-a-chip systems, AI, and computational toxicology are revolutionizing drug development by replacing traditional animal tests. These innovations reduce costs and improve human safety outcomes by providing more relevant biological data.
These technologies’ intersection with regulatory science incentivizes pharmaceutical companies to adopt humane alternatives. This convergence of tech and policy is reshaping how drugs are tested, offering a glimpse into a more ethical and efficient future for biomedical research.
Historical Context of Animal Testing in Drug Development

Animal testing has been a regulatory cornerstone since the 1938 Federal Food, Drug, and Cosmetic Act mandated it for safety evaluation. However, recent legislative changes, starting with FDA Modernization Act 2.0 in 2022, have begun dismantling mandatory animal testing for investigational drugs.
This gradual shift reflects decades of scientific progress and ethical reconsideration, culminating in current efforts to institutionalize non-animal methodologies. Understanding this historical trajectory highlights how entrenched practices evolve toward more humane and scientifically valid approaches.
Contrarian Perspectives and Challenges Ahead

Not all stakeholders embrace the rapid transition away from animal testing. Some industry experts express concerns about the pace of validation and reliability of non-animal methods across diverse drug types.
Challenges remain in standardizing New Approach Methodologies and ensuring regulatory acceptance without compromising safety. Balancing innovation with caution is critical, as premature adoption could risk public health, underscoring the complexity of reforming entrenched scientific and regulatory paradigms.
Toward a Transparent and Humane Future

The convergence of congressional demands, FDA policy shifts, and technological advances drive a transformative era in animal research. Full disclosure of animal use is becoming a cornerstone of accountability and public trust, while regulatory and legislative reforms are accelerating the adoption of humane alternatives.
These changes promise to reshape biomedical research, enhancing ethical standards and scientific rigor. As this reckoning unfolds, the future points toward a more transparent, humane, and innovative research landscape that respects animal welfare and human health.







